United States, New York, Nov. 18, 2024 (GLOBE NEWSWIRE) — Stem Cell Therapy Market
Stem Cell Therapy Market to Reach 31.33 billion, Globally, by 2032 at 11.20% CAGR: Introspective Market Research
Stem Cell Therapy Market is an innovative medical treatment that leverages the regenerative potential of stem cells to repair damaged tissues, treat diseases, and even combat aging processes. Stem cells are unique because they have the potential to differentiate into various types of cells, including muscle, bone, and nerve cells. Several types of stem cells are used in therapies: embryonic stem cells, adult stem cells, and induced pluripotent stem cells (iPSCs), each with distinct applications and benefits.
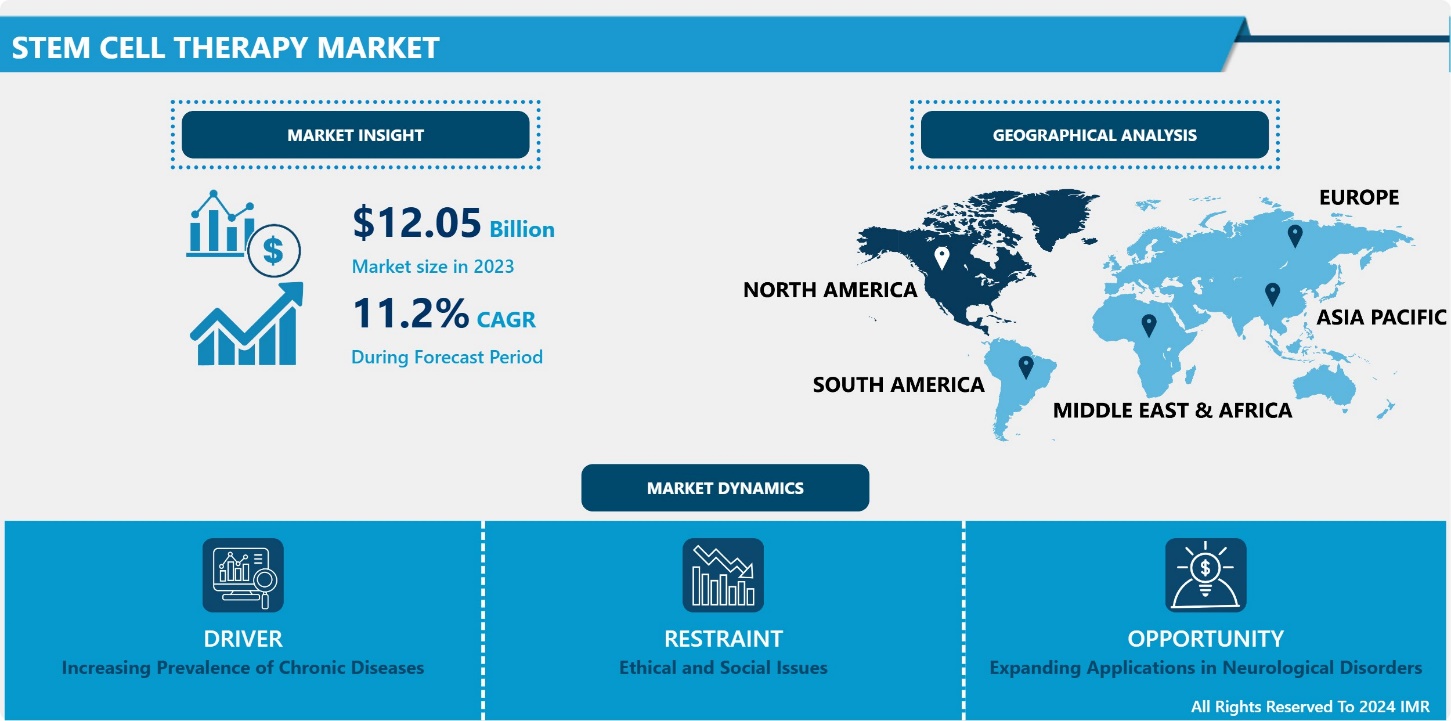
Introspective Market Research is excited to unveil its latest report, “Stem Cell Therapy.” This in-depth analysis shows that the global Stem Cell Therapy Market, valued at USD 12.5 billion in 2023, is poised for substantial growth and is expected to hit USD 31.33 billion by 2032. This growth trajectory aligns with a strong CAGR of 11.20% during the forecast period from 2024 to 2032.
The stem cell therapy market is experiencing rapid growth due to increasing advancements in regenerative medicine, rising investments in research, and a growing demand for innovative treatments targeting chronic and degenerative diseases. Stem cells have unique regenerative properties that allow them to develop into various cell types, offering promising treatment options for conditions like Parkinson’s disease, diabetes, and cardiovascular disorders, where traditional therapies often fall short. This demand is further propelled by the global rise in age-related diseases and conditions.
Regulatory support and an expanding number of clinical trials are boosting market confidence and investment. Technological advancements in stem cell harvesting, cryopreservation, and tissue engineering are enhancing the efficacy and accessibility of stem cell treatments. Increasing consumer awareness about personalized medicine, combined with support from governments and private sectors in the form of funding and favorable policies, is contributing significantly to the market’s expansion. Ethical and regulatory challenges persist, which the industry is addressing through transparent research and collaboration with regulatory bodies, further paving the way for sustained growth in the stem cell therapy market.
Download Sample 250 Pages of Stem Cell Therapy Market Report@ https://introspectivemarketresearch.com/request/17775
Leading Factors Driving the Stem Cell Therapy Market:
Growing Adoption of Regenerative Medicines
The growing adoption of regenerative medicine is a key driver in the expansion of the stem cell therapy market, with regenerative medicine’s promise to restore or replace damaged cells, tissues, and organs leading to transformative advancements in healthcare. Stem cell therapy, one of the most dynamic branches of regenerative medicine, is poised to address many conditions, from chronic diseases to traumatic injuries. This progress is underpinned by stem cells’ unique capabilities for self-renewal and differentiation, allowing them to repair or regenerate tissues.
The rising prevalence of chronic diseases and degenerative conditions, such as diabetes, Parkinson’s disease, and cardiovascular diseases, where conventional treatments often fail to deliver lasting results. Stem cell therapies offer a potential solution, with treatments increasingly demonstrating promising outcomes in clinical trials, attracting attention from healthcare providers and patients alike. As regenerative medicine continues to advance, stem cell therapy is seeing increased acceptance in both clinical and research settings, driving demand.
Regenerative medicine also benefits from advances in biotechnology, which enhance the efficacy, safety, and scalability of stem cell treatments. Recent developments in gene editing and tissue engineering are making stem cell therapies more precise and adaptable to individual patient needs, an essential shift as the market moves toward personalized medicine. These innovations reduce risks associated with traditional therapies, enhancing the appeal and adoption of stem cell interventions.
Investment and supportive regulatory environments are also critical in propelling this market forward. Governments and private entities are investing in research and development, while regulatory bodies worldwide are gradually introducing frameworks to ensure the safety and efficacy of stem cell products. Programs like the FDA’s Regenerative Medicine Advanced Therapy (RMAT) designation, which facilitates expedited approval processes, highlight the regulatory support for these therapies.
The intersection of rising healthcare demand, technological advancements, supportive regulatory policies, and increasing investment is solidifying stem cell therapy as a central component in regenerative medicine’s future. As adoption continues to grow, the stem cell therapy market is anticipated to experience sustained growth, reshaping how chronic and degenerative conditions are managed and positioning regenerative medicine as a revolutionary field in modern healthcare.
“Research made simple and affordable – Trusted Research Tailored just for you – IMR Knowledge Cluster”
https://www.imrknowledgecluster.com/
What are the opportunities in the Stem Cell Therapy Market?
Expanding Applications in Neurological Disorders
Stem cell therapy is increasingly recognized for its transformative potential in treating a range of neurological disorders, representing a critical opportunity for growth in the market. Neurological disorders like Parkinson’s disease, Alzheimer’s disease, multiple sclerosis (MS), and spinal cord injuries pose significant health challenges worldwide. Traditional treatments for these conditions focus largely on symptom management, offering limited potential for recovery or reversal of the damage. Stem cell therapy introduces the promise of regenerative medicine by aiming to repair or replace damaged neurons, potentially restoring lost functions and providing a pathway to significantly better outcomes.
The versatility of stem cells in differentiating into various cell types makes them ideal candidates for treating the complex cellular degradation observed in neurological conditions. For instance, in Parkinson’s disease, stem cells can be induced to become dopamine-producing neurons, addressing the fundamental cause of the disease rather than merely alleviating symptoms. In spinal cord injuries, stem cells may encourage the regeneration of neurons and glial cells, aiding in functional recovery.
Recent advancements in induced pluripotent stem cells (iPSCs) and mesenchymal stem cells (MSCs) offer new hope, as these cells can be sourced from the patient’s own body, reducing the risk of immune rejection and ethical concerns tied to embryonic stem cells. Additionally, iPSCs provide a valuable tool for developing personalized treatments, making therapies more precise and likely to succeed.
Governments and research institutions globally are increasing their funding for stem cell research, particularly in the neurological field, which further drives innovation and accessibility. Clinical trials are already underway for a variety of neurological conditions, and early results indicate promising therapeutic efficacy. Regulatory bodies such as the FDA are establishing frameworks to fast-track stem cell therapies for serious conditions, propelling market growth.
The rising prevalence of neurological disorders, combined with advancements in stem cell technology and a supportive regulatory environment, is creating a unique market opportunity. As the focus on regenerative medicine intensifies, stem cell therapies could emerge as standard interventions for neurodegenerative diseases, leading to significant market expansion.
Ethical and Social Issues hamper Market Growth
Stem cell therapy, a promising field within regenerative medicine, holds immense potential for treating diseases ranging from neurological disorders to organ failure. The foremost ethical concern involves the source of stem cells. Embryonic stem cells (ESCs), derived from human embryos, are highly potent for regenerative applications, but their use raises profound ethical issues. Many views the destruction of embryos for research as morally unacceptable, associating it with potential life termination. This controversy has led to stringent regulations in numerous countries, limiting research funding and impeding the development of ESC-based therapies. Even alternative sources like induced pluripotent stem cells (iPSCs), which do not require embryos, still provoke ethical questions about genetic manipulation and long-term health impacts.
Privacy concerns also arise within stem cell research and treatment. The collection of patient cells, DNA, and health data carries risks of misuse, unauthorized data sharing, and discrimination, especially in the insurance and employment sectors. Ethical guidelines are required to safeguard patient data and establish consent processes that protect patient rights.
Social issues, including unequal access, add another layer of complexity. Stem cell therapy remains expensive, making it inaccessible to low-income populations. This economic disparity has sparked debates around healthcare inequality, raising questions about who can afford and benefit from these advanced treatments. Additionally, the commercialization of stem cell therapy can lead to clinics prioritizing profits over patient welfare, creating a landscape where unproven, high-cost treatments are offered with insufficient regulatory oversight.
Public perception of stem cell therapy, shaped by these ethical and social concerns, further hampers market growth. Misinformation about stem cell applications and potential side effects adds to public skepticism, reducing demand and slowing adoption. Moreover, ethical debates continue to delay supportive legislation, hindering research progression and commercial application.
Do you need any industry insights on Stem Cell Therapy Market, Make an enquiry now >>? https://introspectivemarketresearch.com/inquiry/17775
Key Manufacturers
Market key players and organizations within a specific industry or market that significantly influence its dynamics. Identifying these key players is essential for understanding competitive positioning, market trends, and strategic opportunities.
- Athersys Inc. (U.S.)
- Mesoblast Ltd (Australia)
- Biorestorative Therapies Inc. (U.S.)
- Pluristem Inc. (Israel)
- Brainstorm Cell Limited. (U.S.)
- Viacyte Inc. (U.S.)
- Gamida Cell (U.S.)
- Hope Biosciences (U.S.)
- Cellular Biomedicine Group (U.S.)
- Smith+Nephew (U.K.)
- Medipost (South Korea)
- Anterogen. C.O., Ltd. (South Korea)
- Nuvasive Inc. (U.S.)
- Rti Surgical (U.S.)
- Allosource (U.S.)
- Jcr Pharmaceuticals Co. Ltd. (Japan)
- Takeda Pharmaceutical Company Limited (Japan)
Recent Industry Development
In April 2024: The Agency for Science, Technology, and Research in Singapore and SCG Cell Therapy (SCG) collaborated to advance the manufacturing of induced pluripotent stem cells (iPSC) that meet good manufacturing practices (GMP) standards.
Key Segments of Market Report
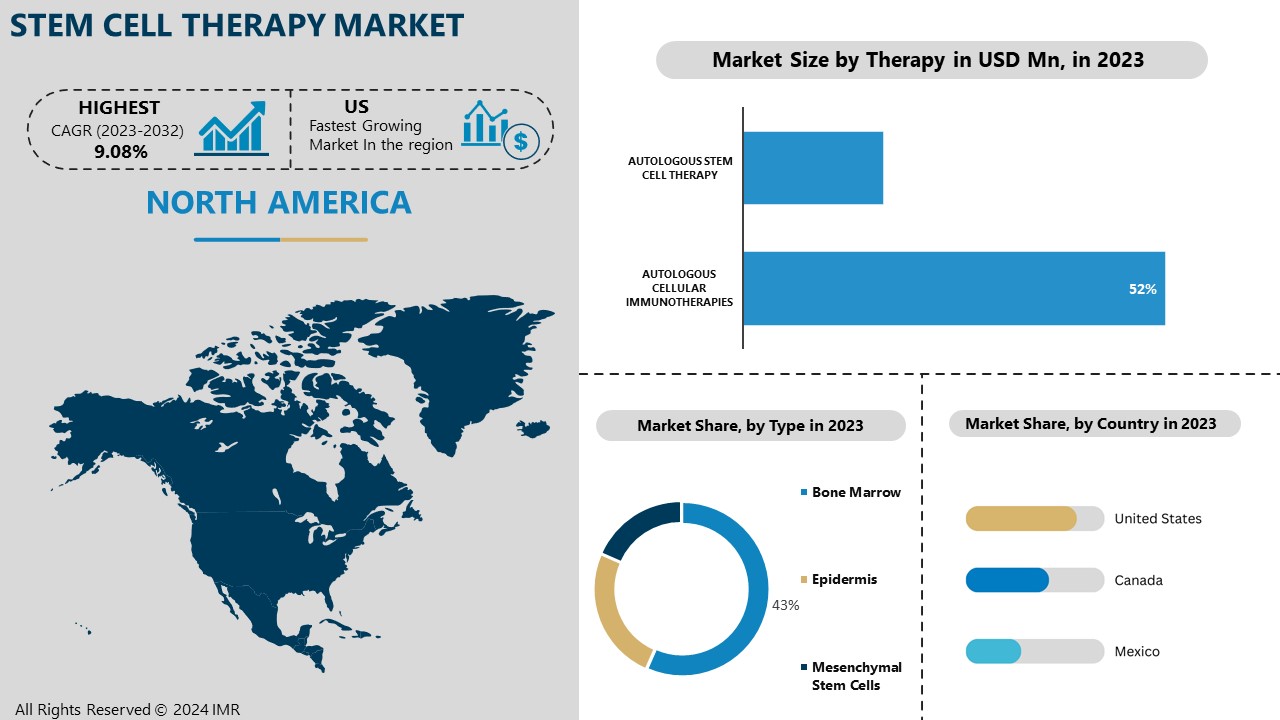
By Therapy, Autologous Cellular Immunotherapies segment is expected to dominate the market during the forecast period
The Autologous Cellular Immunotherapies segment is projected to be a leading driver in the stem cell therapy market over the coming years. This dominance can be attributed to several key factors, particularly the promising therapeutic potential of autologous cell-based treatments in tackling a wide range of chronic and debilitating diseases. Autologous therapies use a patient’s cells, which are extracted, modified, and then reintroduced into the body. This approach minimizes the risk of immune rejection, making it an attractive option for personalized medicine.
The autologous cellular immunotherapies are their high efficacy in treating cancers, autoimmune diseases, and degenerative conditions. For Instance, autologous therapies like CAR-T cell treatments have shown remarkable success in hematologic cancers, leading to durable remissions in patients with limited alternatives. These therapies are increasingly being investigated for solid tumors, further expanding their scope in oncology.
Advances in biotechnology and genetic engineering are also fueling the growth of the autologous cellular immunotherapies segment. Improved cell processing techniques, gene-editing tools like CRISPR, and developments in artificial intelligence have made it easier to modify and enhance autologous cells to target specific disease markers. This has led to more effective treatments and increased patient survival rates, making the segment a focus for ongoing research and development efforts.
As the demand for regenerative and curative treatments rises, healthcare providers and patients are increasingly seeking therapies that offer long-term benefits and fewer side effects compared to traditional treatments. Autologous therapies align well with this trend, providing a patient-centered, personalized approach to treatment.
By Application, Bone Marrow segment held the largest share in 2023
Bone marrow therapy remains one of the most established and widely adopted applications in the stem cell field, owing to its extensive use in treating a variety of hematological diseases, such as leukemia, lymphoma, and multiple myeloma. This therapy has been in clinical practice for several decades, primarily in bone marrow transplants, which are now termed hematopoietic stem cell transplants (HSCTs). The success and efficacy of HSCTs in managing and curing specific blood-related diseases have significantly boosted the market demand for bone marrow stem cell therapies.
The increasing prevalence of hematological and genetic disorders globally has driven healthcare providers to adopt advanced treatments, including stem cell therapies, to improve patient outcomes. The rising cases of immune-related conditions, which often require bone marrow stem cells for immune system rebuilding, have further propelled this segment. Bone marrow stem cells are unique due to their pluripotent nature, which allows them to differentiate into various cell types, making them suitable for treating not only blood-related disorders but also other diseases such as autoimmune disorders.
The regulatory support and funding for bone marrow stem cell therapies also contribute to this segment’s prominence. Many government and private entities worldwide have recognized the efficacy of bone marrow-based stem cell therapy and have invested in research and development (R&D) to expand its applications. For instance, initiatives for improving donor registries and making bone marrow transplants accessible to a broader population, especially in regions where healthcare infrastructure is improving, are fueling market growth.
If you require any specific information that is not covered currently, we will provide the same as a part of the customization >> https://introspectivemarketresearch.com/custom-research/17775
Stem Cell Therapy Market Regional Insights:
North America is Expected to Dominate the Market Over the Forecast period
North America is projected to dominate the global stem cell therapy market, due to advanced healthcare infrastructure, high research and development investments, and a supportive regulatory environment. The United States, in particular, leads this growth due to its well-established medical research facilities, collaborations between government institutions and private companies, and strong funding for biomedical research. The region’s proactive stance on innovative treatments and therapies has led to an increasing number of clinical trials focused on stem cell applications, further accelerating market development.
The North American market benefits from a robust regulatory framework managed by agencies like the U.S. Food and Drug Administration (FDA), which ensures the safety and efficacy of stem cell therapies. Although regulatory hurdles exist, particularly surrounding ethical considerations and safety issues, the FDA’s regenerative medicine policies encourage clinical trials, providing a pathway for promising stem cell therapies to reach the market. This structured yet supportive environment enables the development and approval of novel stem cell treatments for diseases such as cancer, neurodegenerative disorders, and cardiovascular conditions, where conventional treatments may have limited effectiveness.
The high prevalence of chronic diseases in North America has increased demand for advanced treatment options, with stem cell therapy being one of the most promising areas. Chronic conditions like diabetes, Alzheimer’s, and heart disease are widespread in the U.S. and Canada, creating a pressing need for innovative treatments. Stem cell therapy’s potential to regenerate damaged tissues and cells aligns well with this demand, driving market growth.
Strong private and public sector investment fuels advancements in the stem cell therapy field. Major pharmaceutical companies and biotechnology firms in North America are heavily investing in research to develop and commercialize new therapies, while governmental bodies provide grants to support academic and industry collaborations. These investments enable a continuous pipeline of stem cell-based treatments, positioning North America as a leader in the global market.
Comprehensive Offerings:
- Historical Market Size and Competitive Analysis (2017–2023): Detailed assessment of market size and competitive landscape over the past years.
- Historical Pricing Trends and Regional Price Curve (2017–2023): Analysis of historical pricing data and price trends across different regions.
- Market Size, Share, and Forecast by Segment (2024–2032): Projections and detailed insights into market size, share, and future growth by segment.
- Market Dynamics: In-depth analysis of growth drivers, restraints, opportunities, and key trends, with a focus on regional variations.
- Market Trend Analysis: Evaluation of emerging trends that are shaping the market landscape.
- Import and Export Analysis: Examination of trade patterns and their impact on market dynamics.
- Market Segmentation: Comprehensive analysis of market segments and sub-segments, with a regional breakdown.
- Competitive Landscape: Strategic profiles of key players across regions, including competitive benchmarking.
- PESTLE Analysis: Evaluation of the market through Political, Economic, Social, Technological, Legal, and Environmental factors.
- PORTER’s Five Forces Analysis: Assessment of competitive forces influencing the market.
- Industry Value Chain Analysis: Examination of the value chain to identify key stages and contributors.
- Legal and Regulatory Environment by Region: Analysis of the legal landscape and its implications for business operations.
- Strategic Opportunities and SWOT Analysis: Identification of lucrative business opportunities, coupled with a SWOT analysis.
- Conclusion and Strategic Recommendations: Final insights and actionable recommendations for stakeholders.
Related Report Links:
Personalized Cell Therapy Market: Personalized Cell Therapy Market Size Was Valued at USD 15.1 Billion in 2023, and is Projected to Reach USD 114.63 Billion by 2032, Growing at a CAGR of 25% From 2024-2032.
Cell Therapy Market: Cell Therapy Market Size Was Valued at USD 14.6 Billion in 2023, and is Projected to Reach USD 77.6 Billion by 2032, Growing at a CAGR of 20.4% From 2024-2032.
Cell Therapy Manufacturing Market: Cell Therapy Manufacturing Market Size Was Valued at USD 4.5 Billion in 2023, and is Projected to Reach USD 16.1 Billion by 2032, Growing at a CAGR of 15.2% From 2024-2032.
Viral Vector and Plasmid DNA Manufacturing Market: Viral Vector and Plasmid DNA Manufacturing Market Size Was Valued at USD 5.4 Billion in 2023, and is Projected to Reach USD 28.9 Billion by 2032, Growing at a CAGR of 20.9% From 2024-2032.
Mucopolysaccharidosis System Market: Mucopolysaccharidosis System Market Size is Valued at USD 10.79 Billion in 2023, and is Projected to Reach USD 21.39 Billion by 2032, Growing at a CAGR of 7.9% From 2024 to 2032.
Scaffold Technology Market: Scaffold Technology Market Size Was Valued at USD 2.0 Billion in 2023, and is Projected to Reach USD 6.6 Billion by 2032, Growing at a CAGR of 14.2% From 2024-2032.
Red Biotechnology Market: Red Biotechnology Market Size Was Valued at USD 570.2 Billion in 2023, and is Projected to Reach USD 1412.0 Billion by 2032, Growing at a CAGR of 10.60% From 2024-2032.
Biologics Market: Biologics Market Size is Valued at USD 561.75 Billion in 2024 and is Projected to Reach USD 1230.70 Billion by 2032, Growing at a CAGR of 10.3% From 2024-2032.
Regenerative Medicine Market: Regenerative Medicine Market Size Was Valued at USD 22.32 Billion in 2023 and is Projected to Reach USD 137.53 Billion by 2032, Growing at a CAGR of 22.39% From 2024-2032
Cell Culture Market: Cell Culture Market Size Was Valued at USD 18.5 Billion in 2023, and is Projected to Reach USD 48.9 Billion by 2032, Growing at a CAGR of 11.4% From 2024-2032.
About Us:
Introspective Market Research is a premier global market research firm, leveraging big data and advanced analytics to provide strategic insights and consulting solutions that empower clients to anticipate future market dynamics. Our team of experts at IMR enables businesses to gain a comprehensive understanding of historical and current market trends, offering a clear vision for future developments.
Our strong professional network with industry-leading companies grants us access to critical market data, ensuring the generation of precise research data tables and the highest level of accuracy in market forecasting. Under the leadership of CEO Mrs. Swati Kalagate, who fosters a culture of excellence, we are committed to delivering high-quality data and supporting our clients in achieving their business goals.
The insights in our reports are derived from primary interviews with key executives of top companies in the relevant sectors. Our robust secondary data collection process includes extensive online and offline research, coupled with in-depth discussions with knowledgeable industry professionals and analysts.
Contact Us:
Canada Office
Introspective Market Research Private Limited, 138 Downes Street Unit 6203- M5E 0E4, Toronto, Canada.
APAC Office
Introspective Market Research Private Limited, Office No. 401, Saudamini Commercial Complex, Kothrud, Pune, India 411038
Ph no: +91-81800-96367 / +91-7410103736
Email: sales@introspectivemarketresearch.com
LinkedIn| Twitter| Facebook | Instagram
Ours Websites : https://introspectivemarketresearch.com | https://imrknowledgecluster.com/knowledge-cluster | https://imrtechsolutions.com | https://imrnewswire.com/ | https://marketnresearch.de |

