United States, Phoenix, Nov. 26, 2024 (GLOBE NEWSWIRE) — Glenmark Pharmaceuticals announced that it received final approval from the United States Food and Drug Administration (U.S. FDA) for its 0.03% Tacrolimus Ointment, this ointment is a generic version of Protopic Ointment, 0.03%, by Leo Pharma AS. Glenmark Pharmaceuticals Inc., USA will distribute the ointment in the United States.
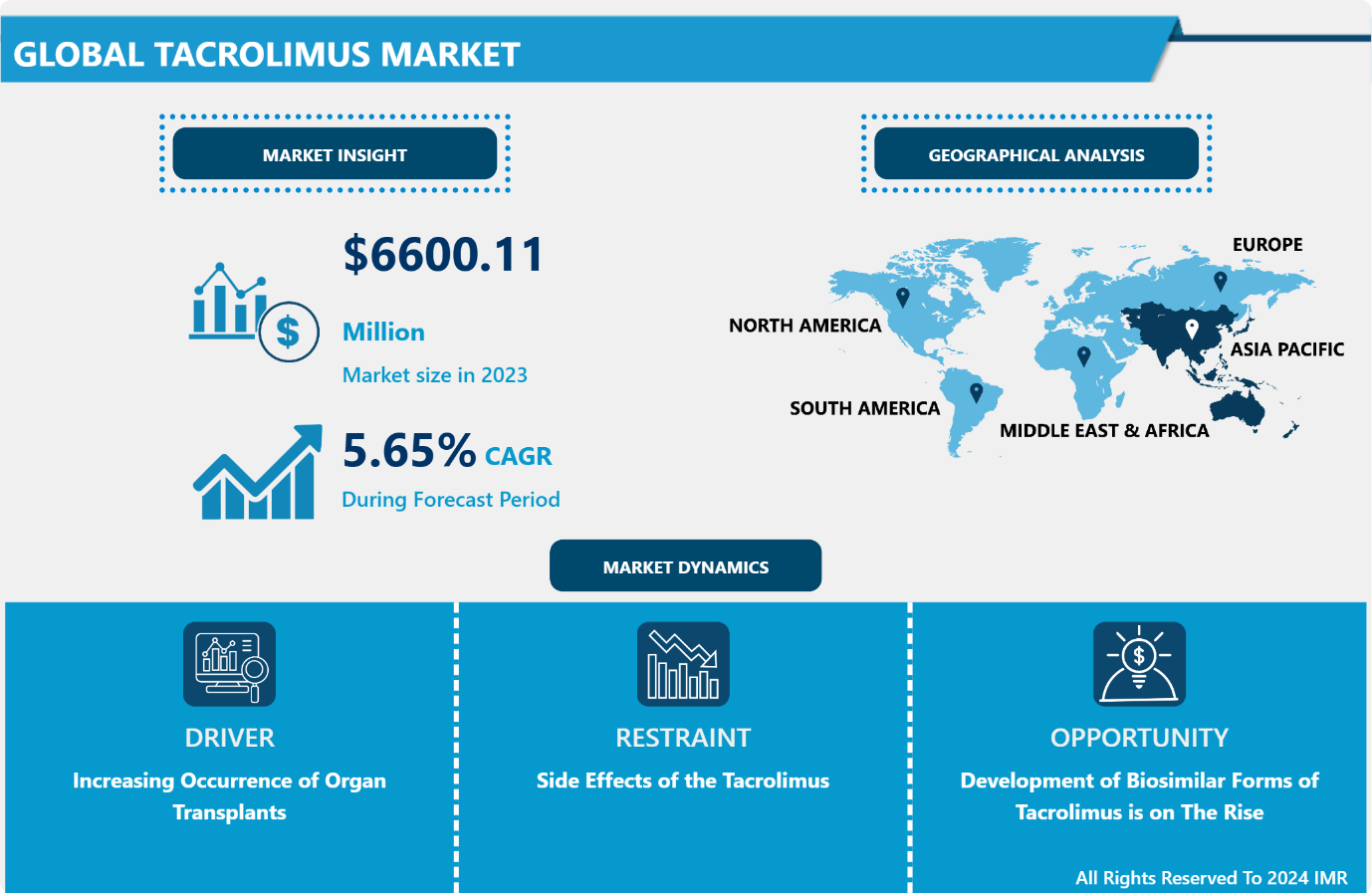
Introspective Market Research is excited to unveil its latest report, Tacrolimus Market This in-depth analysis shows that the global Tacrolimus market, valued at USD 6600.11 million in 2023, is poised for substantial growth, expected to hit USD 10823.72 million by 2032. This growth trajectory aligns with a strong CAGR of 5.65% during the forecast period from 2024 to 2032.
Tacrolimus, derived from Streptomyces tsukubaensis, is a powerful immunosuppressant. It consists of a 23-member macrolide lactone, with a molecular weight of 803.5 Da, in terms of structure. It has also been discovered that Tacrolimus is metabolized by CYP3A4. Lampen et al. showed that both human liver and small intestinal microsomes metabolize tacrolimus, producing similar metabolite patterns in vitro. Research on immunoinhibition showed that blocking CYP3A reduced the production of tacrolimus metabolites. The formation of Tacrolimus metabolite in human intestinal microsomes had a five-fold variation. The production of Tacrolimus metabolites in pig duodenal mucosa, used as a model for human intestinal metabolism, differs based on the gastrointestinal tract section. The gastric mucosa did not produce any tacrolimus metabolites. Lampen et al. observed a prominent variance in the rates of tacrolimus metabolite formation between men and women in duodenal samples from humans.
Tacrolimus is a stronger immunosuppressant than cyclosporine, with 10 to 100 times more potency. Its higher potency and lower molecular weight indicate that it could be a more effective topical treatment compared to cyclosporine. Despite not being chemically related to cyclosporine, tacrolimus works in a similar way to suppress the immune response by blocking the movement of certain molecules within the cell and reducing the production of specific genes that activate T-cells. The precise way in which tacrolimus works in atopic dermatitis is not understood. Tacrolimus stops the activation of T-lymphocytes by initially attaching to the intracellular protein FK506 binding protein 12 (FKBP-12), creating a compound with FKBP-12, calcium, calmodulin, and calcineurin, which hinders the phosphatase activity of calcineurin. It is thought to inhibit the production of lymphokines like interleukin (IL)-2 and interferon-γ. Tacrolimus also hinders the production of genes responsible for IL-3, IL-4, IL-5, GM-CSF, and TNF-α; prevents the release of preexisting mediators from skin mast cells and basophils; and decreases FcɛRI expression on Langerhans cells.
Tacrolimus is used to manage skin inflammation, especially atopic dermatitis when topical corticosteroids and moisturizers are ineffective. It suppresses annoyance similar to steroids and is as effective as average-quality steroids. One important benefit of tacrolimus is that, unlike steroids, it does not result in skin thinning or other side effects typically seen with steroids. It can be used continuously in small amounts (twice a week) on delicate facial skin and eyelids, even during the healing process of injuries. Clinical trials were carried out for a maximum of one year, Tacrolimus is beneficial in managing atopic dermatitis in children (aged 2 years and above) as well as adults. The primary side effect of tacrolimus is commonly itching and a burning feeling on the skin where it is used. Rare systemic issues occur due to the minimal absorption of this sizable molecule.
Download Sample 250 Pages of Acrylic Adhesives Market Report@ https://introspectivemarketresearch.com/request/16966
Leading Factors Driving the Tacrolimus Market:
Increasing Occurrence of Organ Transplants
Tacrolimus is given to allograft recipients undergoing immunosuppressive drug therapy, it is crucial in organ transplantation procedures. In 2023, a global total of 46,632 organ transplants were carried out, as reported by the Global Observatory on Donation and Transplantation. Similarly, In 2023, the Australian and New Zealand Organ Donor Registry (ANZOD) reported significant transplant activity: Australia witnessed 1,396 organ transplant recipients, resulting in 1,588 organs transplanted from deceased donors, while New Zealand recorded 195 recipients and 213 organs transplanted. The growing demand for immunosuppressive therapies like tacrolimus is closely linked to this upward trend, ensuring successful transplant outcomes and improving patient survival rates. In Australia, kidneys accounted for the majority of transplants (838), followed by livers (268) and lungs (304). Similarly, New Zealand saw kidneys leading with 101 transplants, alongside 55 liver and 25 lung transplants. The increasing need for these procedures underscores the importance of tacrolimus in post-transplant care, driving its adoption as a cornerstone therapy. This sustained demand is expected to fuel market growth, supported by advancements in transplantation techniques and growing awareness of organ donation.
Tacrolimus, a potent immunosuppressive agent, is crucial in transplant medicine as it suppresses the recipient’s immune response to the transplanted organ. Tacrolimus helps prevent the immune system from attacking the newly transplanted organ by inhibiting T-cell activation and cytokine production, reducing the risk of rejection. Tacrolimus provides advantages like improved effectiveness and reduced rate of acute rejection as compared to previous immunosuppressive drugs. Its ability to achieve effective immunosuppression while reducing negative side effects has established it as a key element in post-transplant immunosuppressive treatments. With the increasing adoption of transplant procedures worldwide, such an increase in the number of transplant procedures is expected to boost the acceptance of tacrolimus medications, in turn, driving the market growth over the forecast period.
Development of Biosimilar Forms of Tacrolimus is on The Rise
The development of biosimilars generally leads to reduced research and development expenses in comparison to original drugs, allowing manufacturers to sell these products at a more affordable price. This address concerns regarding healthcare systems, safeguards, and patients in areas with limited healthcare funding or where tacrolimus treatment may be prohibitively expensive. The effectiveness and safety profiles of biosimilar tacrolimus products are maintained at comparable levels to the original brand formulation. Administrative organizations procure biosimilars to demonstrate similarity in quality, safety, and effectiveness to the reference product through comprehensive comparative studies. This ensures that patients can trust the healing effectiveness of biosimilar tacrolimus, ensuring successful immune system suppression treatment after transplantation.
The availability of biosimilar tacrolimus products also promotes market competition, leading to lower costs and increased accessibility. Rivalry among biosimilar manufacturers leads to progress, efficiency, and cost-cutting measures, ultimately benefiting patients and healthcare systems. Rising competition encourages a steady stream of innovation that will push individual producers to improve their products or adjust pricing strategies to remain competitive. Biosimilar tacrolimus products show potential in expanding market access, especially in areas with limited access to branded products or regulatory barriers inhibiting market entry. Biosimilars have the potential to improve access to essential medications, promote treatment adherence, and reduce healthcare inequalities by making tacrolimus therapy more accessible and cost-effective.
“Research made simple and affordable – Trusted Research Tailored just for you – IMR Knowledge Cluster”
https://www.imrknowledgecluster.com/
What are the Key Opportunities for the Tacrolimus Market?
With the increasing demand for organ transplants caused by the rise in organ failure from chronic diseases, the market for Tacrolimus is anticipated to expand. Developments in transplant techniques and increased knowledge are expanding the application of Tacrolimus as an essential treatment for suppressing the immune system. Moreover, the increasing occurrence of autoimmune diseases like rheumatoid arthritis and psoriasis is expanding the drug’s usage, enhancing market prospects.
Emerging economies in nations such as India, China, and Brazil offer profitable chances because of the expansion in healthcare facilities and backing from the government. Greater availability of generic Tacrolimus is now accessible to more patients in regions where costs are a concern due to patent expiration, resulting in improved accessibility of this crucial medication.
Innovations in drug forms such as slow-release capsules and skin creams are improving patient adherence and treatment effectiveness. Exploring new delivery methods, such as those using nanotechnology, and broadening the uses for skin conditions such as eczema and atopic dermatitis could result in potential market expansion.
The expanded use of online health platforms and web pharmacies is widening the availability of Tacrolimus, especially in rural regions. Additionally, the move towards personalized medicine is anticipated to improve the effectiveness of Tacrolimus with customized treatments, leading to a broader market penetration. The combination of these factors will lay the groundwork for strong growth in the Tacrolimus market over the next few years.
What are the Key Challenges Tacrolimus Market?
The substantial upfront investment required for developing and installing Tacrolimus solutions, particularly for large-scale projects, constitutes a significant barrier to market growth. Challenges related to intermittency and reliability, particularly evident in some Tacrolimus solutions like solar and wind energy, pose hurdles, especially in regions with erratic weather patterns.
Significantly, infrastructure investments are necessary, including upgrades to the grid and the establishment of storage facilities, to facilitate the seamless integration of Tacrolimus solutions into existing energy systems. Uncertainty surrounding government policies and regulations, such as fluctuations in subsidies or tax incentives, introduces instability for investors, potentially impeding market growth. Established and subsidized technologies like fossil fuels and nuclear energy present formidable competition to the adoption of Tacrolimus solutions, particularly in regions where they are entrenched. Disruptions in the supply chain, such as shortages of critical materials or components, can disrupt the availability and pricing of Tacrolimus solutions, impacting market growth.
Key Manufacturers
Market key players and organizations within a specific industry or market that significantly influence its dynamics. Identifying these key players is essential for understanding competitive positioning, market trends, and strategic opportunities.
- Astellas Pharma Inc. (Japan)
- Fujisawa Pharmaceutical Co., Ltd. (Japan)
- Novartis AG (Switzerland)
- Mylan N.V. (USA)
- Sandoz (a division of Novartis) (Switzerland)
- Dr. Reddy’s Laboratories Ltd. (India)
- Glenmark Pharmaceuticals Ltd. (India)
- Cipla Ltd. (India)
- Zydus Cadila (India)
- Sun Pharmaceutical Industries Ltd. (India)
- Biocon Ltd. (India)
- AbbVie Inc. (USA)
- Pfizer Inc. (USA)
- Roche Holding AG (Switzerland)
- Allergan, Inc. (USA)
- Mylan Pharmaceuticals (USA)
- Teva Pharmaceutical Industries Ltd. (Israel)
- Wockhardt Ltd. (India)
- Torrent Pharmaceuticals Ltd. (India)
- Hikma Pharmaceuticals PLC (UK)
- Intas Pharmaceuticals Ltd. (India)
- Strides Pharma Science Ltd. (India)
- Aurobindo Pharma Ltd. (India)
- Lupin Ltd. (India)
- Alkem Laboratories Ltd. (India)
- Janssen Pharmaceuticals (Johnson & Johnson) (USA)
- Eisai Co., Ltd. (Japan)
- Veloxis Pharmaceuticals (Denmark)
- Bristol-Myers Squibb (USA)
- Apotex Inc. (Canada), and Other Active Players
In April 2024, Biocon has announced that it has received approval from the South African Health Products Regulatory Authority (SAHPRA) for the vertically integrated combination drug Tacrolimus capsule in South Africa.
In September 2023, The Food and Drug Administration (FDA) has changed the therapeutic equivalence classification of Accord Healthcare’s generic tacrolimus capsules from AB to BX, so it is no longer recommended as an automatic substitute for the brand name Prograf (tacrolimus) oral capsules.
Do you need any industry insights on Acrylic Adhesives Market, Make an enquiry now >>? https://introspectivemarketresearch.com/inquiry/16966
Key Segments of Market Report
By Type
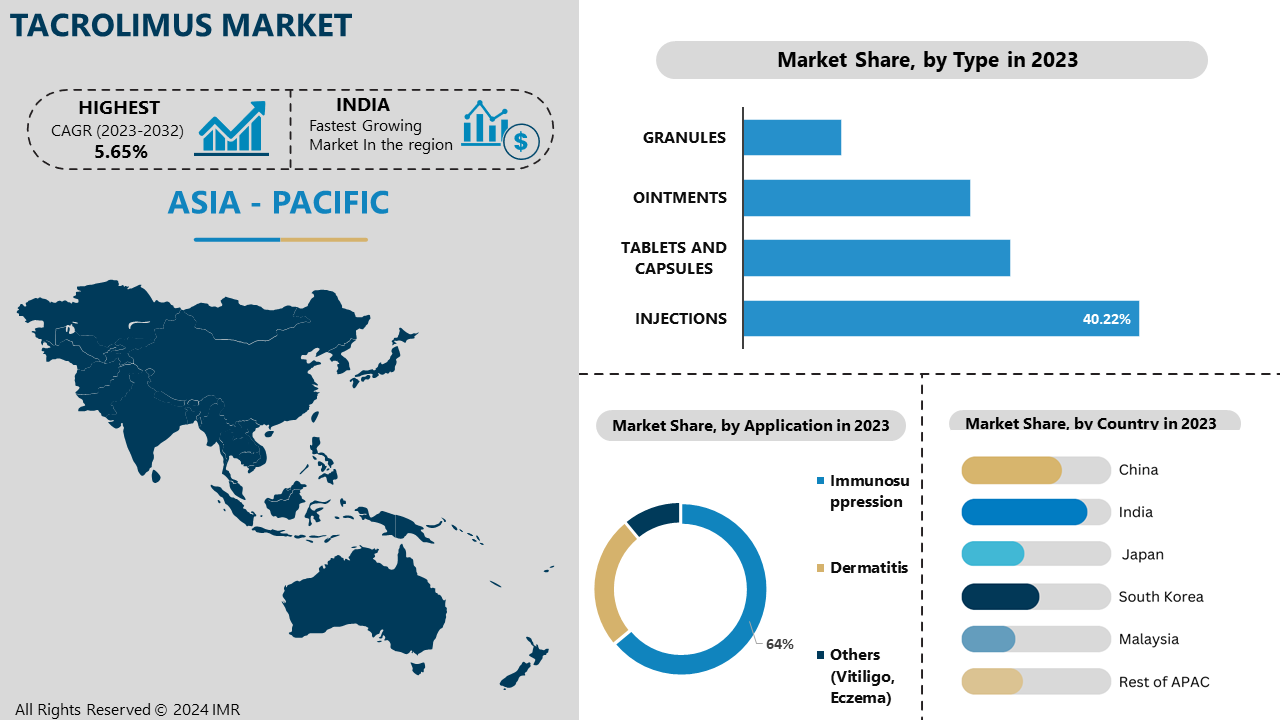
Tacrolimus injections provide quick activation, making them ideal for situations needing immediate immunosuppression, such as during the perioperative period or following transplantation with rapid discharge. Administered via IV or IM, they ensure rapid absorption and transport, leading to prompt beneficial outcomes, including hospitals and organ transplant centers, injections are preferred for their precise dosage and quick therapeutic response. Tacrolimus injections provide healthcare professionals with increased control over dosing regimens, allowing adjustments based on the patient’s clinical condition, and promoting optimal management of immunosuppression in critically ill patients.
Tacrolimus injections ensure effective drug delivery and absorption for patients with gastrointestinal issues or those who cannot tolerate oral medications by skipping the digestive system. This optional program improves compliance and effectiveness in managing treatments, especially when dealing with difficulties in taking oral medications. Tacrolimus injections are frequently used to treat severe rejection episodes following organ transplantation. Their rapid function and high absorption enable quick suppression of the immune response, aiding in reducing inflammation and maintaining joint function, essential for promoting recovery. Injectable medications are often given priority for use by patients in healing center formularies, especially in general care and perioperative environments. Tacrolimus injections are preferred in intensive care situations because of their established effectiveness, safety, and convenience, aligning with hospital preferences and ensuring seamless incorporation into treatment protocols.
By Application
Tacrolimus drugs are utilized for immunosuppression in transplant operations, reducing the body’s capability to reject a donated organ. The part of immunosuppression is expected to experience notable expansion in the coming years due to the rising number of transplants, growth in research and development efforts for immunosuppression, and an increase in the adoption of tacrolimus treatments by patients.
Tacrolimus is recognized for its effectiveness in suppressing the immune system. The ability to specifically inhibit the activation of T-lymphocytes, crucial for the immune response to transplanted organs, enhances its appeal compared to other immunosuppressants. In addition to being used for transplantation, tacrolimus is also used for various immune system disorders such as severe eczema, rheumatoid arthritis, and inflammatory bowel disease in cases where immune suppression is beneficial. This further promotes its advertising potential considerably. Government agencies and medical guidelines consistently support the utilization of tacrolimus as a common immunosuppressive therapy in organ transplant procedures. This approval from experts enhances its strong market position. Innovations in defining tacrolimus, like extended-release options, have enhanced patient compliance and outcomes, enhancing its role in long-term immunosuppressive therapy.
By Region
In the Asia-Pacific region, there is a growing need for organ transplants, leading to an increased usage of tacrolimus as an immunosuppressive medication. Japan, where Astellas Pharma Inc. is located, has a significant influence on the market as one of the first creators and leading manufacturers of tacrolimus. India is home to several key pharmaceutical manufacturers like Dr. Reddy’s Laboratories, Cipla, Sun Pharmaceutical Industries, and others, all playing a role in the production and global distribution of tacrolimus.
Major efforts in research and development by businesses in this area contribute to the improvement of the formulations and applications of tacrolimus, increasing its accessibility and effectiveness. Improvements in the healthcare system and increasing healthcare spending in the Asia-Pacific region have improved the ability to carry out organ transplants and provide post-transplant care, therefore increasing the need for tacrolimus. Positive government policies and administrative mechanisms supporting organ donation and transplantation have significantly contributed to boosting the market in this area.
If you require any specific information that is not covered currently, we will provide the same as a part of the customization >> https://introspectivemarketresearch.com/custom-research/16966
Comprehensive Offerings:
- Historical Market Size and Competitive Analysis (2017–2023): Detailed assessment of market size and competitive landscape over the past years.
- Historical Pricing Trends and Regional Price Curve (2017–2023): Analysis of historical pricing data and price trends across different regions.
- Market Size, Share, and Forecast by Segment (2024–2032): Projections and detailed insights into market size, share, and future growth by segment.
- Market Dynamics: In-depth analysis of growth drivers, restraints, opportunities, and key trends, with a focus on regional variations.
- Market Trend Analysis: Evaluation of emerging trends that are shaping the market landscape.
- Import and Export Analysis: Examination of trade patterns and their impact on market dynamics.
- Market Segmentation: Comprehensive analysis of market segments and sub-segments, with a regional breakdown.
- Competitive Landscape: Strategic profiles of key players across regions, including competitive benchmarking.
- PESTLE Analysis: Evaluation of the market through Political, Economic, Social, Technological, Legal, and Environmental factors.
- PORTER’s Five Forces Analysis: Assessment of competitive forces influencing the market.
- Industry Value Chain Analysis: Examination of the value chain to identify key stages and contributors.
- Legal and Regulatory Environment by Region: Analysis of the legal landscape and its implications for business operations.
- Strategic Opportunities and SWOT Analysis: Identification of lucrative business opportunities, coupled with a SWOT analysis.
- Conclusion and Strategic Recommendations: Final insights and actionable recommendations for stakeholders.
Related Report Links:
Diagnostic Imaging Services Market: Diagnostic Imaging Services Market size is expected to grow from USD 611.3 Billion in 2023 to USD 1041.58 Billion by 2032, at a CAGR of 6.1% during the forecast period (2024-2032).
Healthcare Biometrics Market: Healthcare Biometrics Market Size Was Valued at USD 8.26 Billion in 2023 and is Projected to Reach USD 46.72 Billion by 2032, Growing at a CAGR of 21.23 % From 2024-2032.
Dental 3D Printing Materials Market: Dental 3D Printing Materials Market size is expected to grow from USD 3.57 Billion in 2023 to USD 15.93 Billion by 2032, at a CAGR of 18.08 % during the forecast period (2024-2032).
Fertility Testing Devices Market: Global Fertility Testing Devices Market Size Was Valued at USD 702.59 Million In 2023 And Is Projected to Reach USD 1171.55 Million By 2032, Growing at A CAGR of 6.6% From 2024 To 2032.
Infusion Pump Market: The Infusion Pump Market size is estimated at 14.61 billion USD in 2023 and is expected to reach 21.44 billion USD by 2032, growing at a CAGR of 4.9% during the forecast period (2024-2032).
Medical Kits Market: The Global Market for Medical Kits Estimated at USD 207.36 Million In the Year 2023, Is Projected To Reach A Revised Size Of USD 335.74 Million By 2032, Growing At A CAGR Of 5.50% Over The Forecast Period 2024-2032
Wheelchairs Market: The Global Wheelchairs Market size is expected to grow from USD 4 billion in 2023 to USD 5.69 billion by 2032, at a CAGR of 4% during the forecast period (2024-2032).
Surgical Kits Market: Surgical Kits Market Size Was Valued at USD 19.63 Billion in 2023 and is Projected to Reach USD 36.39 Billion by 2032, Growing at a CAGR of 7.10 % From 2024-2032.
Personal Emergency Response System (PERS) Market: The global market for PERS) estimated at USD 9.38 Billion in the year 2023, is expected to reach a revised size of USD 17.39 Billion by 2032, growing at a CAGR of 7.1% over the forecast period 2024-2032.
Robotic Angiography System Market: Robotic Angiography System Market Size Was Valued at USD 679.67 Million in 2023, and is Projected to Reach USD 1262.21 Million by 2032, Growing at a CAGR of 7.12% From 2024-2032.
About Us:
Introspective Market Research is a top global market research company that uses big data and advanced analytics to offer strategic insights and consulting services, enabling clients to predict future market trends. Our group of specialists at IMR helps businesses obtain a thorough understanding of past and present market trends, providing a clear insight into future advancements.
Our extensive professional connections with top companies provide us with essential market data, enabling us to create accurate research tables and achieve the highest level of precision in market prediction. Led by CEO Mrs Swati Kalagate, who promotes a culture of excellence, we are dedicated to providing top-notch data and assisting our clients in reaching their business objectives.
The information in our reports comes from direct interviews with important executives from leading companies in the respective industries. Our thorough secondary data-gathering process involves comprehensive online and offline research, as well as detailed conversations with industry experts and analysts.
Contact Us:
Canada Office
Introspective Market Research Private Limited, 138 Downes Street Unit 6203- M5E 0E4, Toronto, Canada.
APAC Office
Introspective Market Research Private Limited, Office No. 401, Saudamini Commercial Complex, Kothrud, Pune, India 411038
Ph no: +91-81800-96367 / +91-7410103736
Email: sales@introspectivemarketresearch.com
LinkedIn| Twitter| Facebook | Instagram
Ours Websites : https://introspectivemarketresearch.com | https://imrknowledgecluster.com/knowledge-cluster | https://imrtechsolutions.com | https://imrnewswire.com/ | https://marketnresearch.de |
